

Click on a button below to go to the section of your choice.
GENERAL CHEMISTRY
1) Neutrons: Neutrons are uncharged particles that are found in the center of the atom (the nucleus). Neutrons give mass (weight) to the atom but do not participate in chemical reactions.
2) Protons: Protons are positively charged particles that are also found in the nucleus. Like neutrons, protons give mass to the atom but do not participate in chemical reactions. The number of protons an atom has is called the atomís atomic number, and determines the atomís identity (e.g. carbon atoms have 6 protons, oxygen atoms have 8 protons).
3) Electrons: Electrons are negatively charged particles that are found in electron shells surrounding the nucleus. They have essentially no mass but are important in chemical reactions. The first electron shell can hold 2 electrons. For most biologically relevant atoms, additional electron shells (if present) can hold up to eight electrons.
Each atom contains equal numbers of protons and electrons. The number of neutrons may vary within atoms of a particular type. (For example, carbon atoms may have 6, 7, or 8 neutrons). Atoms that vary only in the number of neutrons are called isotopes.
Bonds
Atomic stability:
The stability of atoms depends on whether or not their outer-most shell is filled with electrons. If the outer shell is filled, the atom is stable. Atoms with unfilled outer shells are unstable, and will usually form chemical bonds with other atoms to achieve stability.
|
Example of an unstable atom with a single electron in its outer-most shell. |
|
|
Example of an unstable atom with 7 electrons in its outer-most shell. |
|
|
Examples of stable atoms. |
|
The two types of chemical bonds that atoms can form to achieve stability are called ionic bonds and covalent bonds. In ionic bonds, atoms donate or receive electrons to achieve stability. In covalent bonds, atoms share electrons to achieve stability. The type(s) of bond a particular atom can form depends on the numbers of electrons in their outer shells.
Ionic bonds:
Ionic bonds result from the transfer of electrons between atoms. If an atom has only a few electrons in its outer shell, it can achieve stability by donating these electrons to an atom that has an outer shell that is almost full.
For example:
An atom of sodium has only one electron in its outer shell. An atom of chlorine has seven outer electrons (almost a full shell).
|
|
|
If the lone sodium outer electron is transferred to the chlorine atom, both atoms would have full outer shells and so would achieve stability.
|
|
When clorine gains an extra electron, it is called chloride. |
Since chlorine gained a negatively charged electron, it now has more electrons than protons and has a net negative charge. Since sodium lost an electron, it now has more protons than electrons and so has a net positive charge. Charged particles (like sodium and chlorine after the transfer of electrons) are called ions. (Specifically, positively charged ions are called cations and negatively charged ions are called anions). Due to the opposite charge of the two ions (negative chloride and positive sodium), the two ions are attracted to each other and form an ionic bond. The resulting compound is referred to as an ionic compound. Note that ionic compounds have totally different properties than either of the atoms that make them up. Sodium is a highly reactive silver metal. Chlorine is a toxic green gas. Sodium chloride is table salt.
The animation below further illustrates the formation of the ionic compound sodium chloride.
Ionic compounds can form from the transfer of one or two (occasionally three) electrons.
Note that in the watery environment of cells or solutions, the water molecules disrupt the ionic bonds by keeping the different ions separate. Ions are also called electrolytes. Sodium, chloride, calcium, and potassium are biologically important electrolytes.
Covalent bonds:
Covalent bonds occur when atoms achieve stability by sharing electrons. In the picture below, the central carbon atom is sharing one pair of electrons with 4 different hydrogen atoms, forming a molecule of CH4 (methane). Note that if you count all the electrons in the outer shell of carbon, there are 8, so it is stable. If you count all the electrons in each hydrogen outer shell, there are 2. Since the first shell only holds two electrons, the hydrogen atoms are also stable.
The animation below shows another view of how the atoms in methane share electrons.
It is also possible for atoms to share two or three pairs of electrons. In the picture below, the two oxygen atoms are sharing two pairs of electrons, forming a molecule of oxygen gas (O2).
Covalent bonds are often represented as single lines in chemical formulas. For example, in the covalent compounds shown below, each line represents a shared pair of electrons.
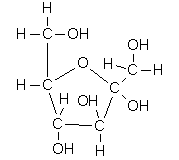
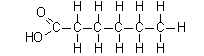
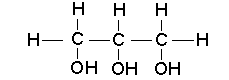
Polar Compounds and Hydrogen Bonds:
Sometimes the atoms in a covalent bond donít share the electrons equally. If that happens, the atom that hogs the electrons more of the time will have a partially negative charge, and the atom that doesnít get the electrons as much will have a partially positive charge. When this happens, it is called a polar covalent bond, and the molecules formed by polar covalent bonds are called polar molecules. Nitrogen and oxygen have a higher affinity for electrons than hydrogen does, so whenever hydrogen atoms are bound to nitrogen or oxygen it is a polar compound. Water (H2O) is an example of a polar compound.
|
|
|
|
Since part of each water molecule is positive and part of the
molecule is negative, adjacent water molecules will tend to be
attracted to each other. The attraction between the partially
positive hydrogen atom of one molecule and the partially negative
oxygen (or nitrogen) atom of another molecule is called a hydrogen
bond.
|
 The attraction between the negative oxygen and the positive hydrogen of different water molecules is an example of a hydrogen bond. |
Hydrogen bonds give water many of its unique properties. Hydrogen bonds also are important in maintaining the three dimensional shape of proteins and holding the strands of DNA together. Hydrogen bonds are much weaker than covalent or ionic bonds.
Hydrogen bonds. In order to understand hydrogen bonds, you must first understand the nature of a special type of covalent bond called the polar covalent bond.
1. In polar covalent bonds, the electrons are more strongly attracted to one of the two atoms, usually oxygen or nitrogen, than to the other atom.
2. Because the negatively charged electron spends more time around the oxygen or nitrogen, these atoms become slightly negatively charged.
3. Because the other atom has the electron less often it is left with a slight positive charge.
Example: Water molecules containpolar covalent bonds because the large oxygen nucleus creates a greater pull on the electron pair than the small nucleus of the hydrogen atom. This produces a partial negative charge around the oxygen while leaving behind a partial positive charge with the hydrogen atom.
Hydrogen bonds always form between a hydrogen atom of one molecule and an oxygen or nitrogen atom of another molecule, if the oxygen or nitrogen is forming a polar covalent bond. The adherence ("stickiness") of water molecules to each other is an excellent example of hydrogen bonding. With water, remember that the molecules are neutral overall but the oxygen is slightly negative and the hydrogens are slightly positive. The hydrogen bonds form between the slightly positive charge of the hydrogen atoms being weakly attracted to the slightly negative charge of the oxygen atom on a different water molecule. As a result, water molecules become stuck to each other, as in the illustrations below. Hydrogen bonds are also found in nucleic acid molecules, DNA and RNA. In DNA molecules, hydrogen bonds between the bases keeping the two strands held together in a double-helix. Hydrogen bonds are also found in transfer RNA molecules as well as between DNA nucleotides and newly formed mRNA strand. The structure of many protein molecules also depend on the formation of hydrogen bonds.
Examples of chemical representations of hydrogen bonds:
| Hydrogen bonds between a guanine nucleotide and a cytosine nucleotide in two separate DNA molecules. |
 |
| Two water molecules forming hydrogen bonds with an adenine molecule. | 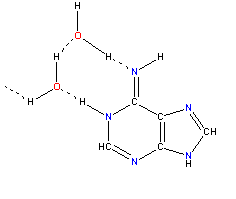 |
| Five water molecules forming hydrogen bonds. The oxygen atoms are blue and the hydrogen atoms are red. The hydrogen bonds are represented by the series of lines between the hydrogen atom of one molecule and the oxygen atom of another molecule. |
|
Before taking the mastery quiz for this topic, be sure to view the following web link to reinforce the topics reviewed in this module: http://www2.nl.edu/jste/bonds.htm
The pH scale measures how many hydrogen ions, H+, are present in a solution. Since concentration means how much of a substance is present in a solution, the pH scale measures hydrogen ion concentration. Because the range in hydrogen ion concentration is extremely large, a logarithmic scale is needed to express the number of hydrogen ions present. Using a logarithmic scale means for each whole number change, the number of hydrogen ions in solution changes by a factor of 10. Low pH values represent the greatest number of hydrogen ions present and large numbers represent the least number of hydrogen ions present.
Water molecules are composed of two hydrogen atoms and one oxygen atom covalently bound together. But, sometimes one of the hydrogen atoms leaves the water molecule as a hydrogen ion. (The hydrogen electron stays with the oxygen.) Consequently, water has a mixture of H+ and OH-. When there is an equal number of H+ and OH-, the value on the pH scale is 7. If there are more H+ than OH- in a solution, the pH scale values are less than 7. If there are less H+ than OH- in a solution, the pH scale values are greater than 7. How does a solution get unequal amounts of H+ and OH-?
USE THIS ANIMATION TO HELP YOU UNDERSTAND pH
YOU WILL NEED FLASH 9.0 TO VIEW THESE CORRECTLY. FOLLOW THE LINK BELOW TO UPDATE YOUR PLUG-IN.
http://www.macromedia.com/software/flash/about/
| Acids are compounds that donate H+ when added to
water.
1. Add one drop of HCl to the water. How did the pH value change? 2. Add additional drops of HCl. How did the pH values change? Bases are compounds that donate OH- when added to water. 3. Without resetting the animation, add one drop of KOH. How did the pH value change? What happened to the visible H+? 4. Add additional drops of KOH. What happened to the visible H+? How did the pH values change? 5. Reset the animation and add several drops of either HCl or KOH to the solution. How does the pH value change? |
A strong acid, such as HCl, completely disassociates. This means that all the acid molecules completely separate into hydrogen ions and anions (e.g. HCl molecules separate into H+ and Cl-). Weak acids, such as H2CO3, do not completely disassociate. Some molecules of the H2CO3 disassociate into H+ and HCO3-, while other molecules remain as H2CO3. Click on the HCl dropper below to see how a strong acid will work in solution. Then click on the H2CO3 dropper to see how this weak acid will disassociate in solution.
|
Common Chemicals |
pH |
| Sodium hydroxide NaOH (lye or caustic soda) | 14.0 |
| Magnesium hydroxide Mg(OH)2 (Milk of Magnesia) | 10.5 |
| Sodium bicarbonate NaHCO3 (Baking Soda) | 8.3 |
| Acetic Acid CH3COOH (in vinegar) | 2.2 |
| Sulfuric Acid H2SO4 (in lead-acid batteries) | 0.5 |
| Hydrochloric acid HCl (1M) | 0.1 |
|
Material |
pH |
Material |
pH |
| Beer | 4.5 | Coffee | 5.0 |
| Apple Juice | 3.5 | Milk | 6.5 |
| Cola | 2.5 | Pure Water | 7.0 |
| Lemon Juice | 2.4 | Human Blood | 7.4 |
| Gastric Juice | 1.7 | Sea Water | 8.0 |
The pH of human blood, cell cytoplasm, and other body fluids is usually in the range of 7.35 to 7.45. Since a pH of 7 is neutral, these fluids can be called weak bases (or more alkaline). For a review of the pH scale see these images:
http://www.epa.gov/acidrain/site_students/images/phscale.gif
http://mddnr.chesapeakebay.net/eyesonthebay/images/ph.gif
http://teacherweb.com/NF/StPetersJuniorHigh/MrGWilliams/ph.bmp
It is easy to change the pH of our blood. Just by holding your breath for a few seconds you can build up CO2 in the body which leads to a greater number of H+ ions in your blood. How would you describe the pH of this blood (more acidic or more basic)? Answer
Another common change in pH occurs when we exercise strenuously and feel sensations of heat (ďthe burnĒ) in our muscles. The increase in acidity in our muscles is responsible for the burn. After a few minutes this burning feeling usually goes away.
As pH changes from its normal range of 7.35-7.45, the body attempts to return pH to its normal range. Molecules in the body can take up excess hydrogen ions (H+) in acidic situations and release hydrogen ions (H+) in basic situations. These molecules are called buffers.

In this cartoon, think of the buffer as a sponge that can grab hydrogen ions from solution or release them. Which direction would this reaction go if the cellís pH was too low (acidic)?
Which direction would this reaction go if the cellís pH was too high (basic)?
Consider a beaker filled with a buffer solution. Observe the following animation that allows you to change the pH of a solution by adding a strong acid or a strong base. The pH of the buffer solution will briefly change and then return to its starting point.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/buffer12.swf
Answer the following questions regarding the animation:
What is the pH of the solution immediately following the addition of HCl? Answer
What is the pH of the solution immediately following the addition of NaOH? Answer
In either case, what happens to the pH of the buffer solution after some time passes? Answer
The animation labels HCl as a strong acid. Would you expect a strong acid to release more H+ in a solution than a weak acid? Compare strong acids with weak acids at Wikipedia. Answer
The animation labels NaOH as a strong base. Would you expect a strong base to accept more protons than a weak base? Answer
The breakdown of amino acid molecules in our cells releases ammonia (NH3). Ammonia can take on a hydrogen ion forming an ammonium ion (NH4+). Review the diagram below.

What would this reaction do to the pH of the cell? Answer
In this example, what term properly describes the NH3? Answer